Can wood substrates be phytotoxic to greenhouse crops?
Added on 16 June 2020
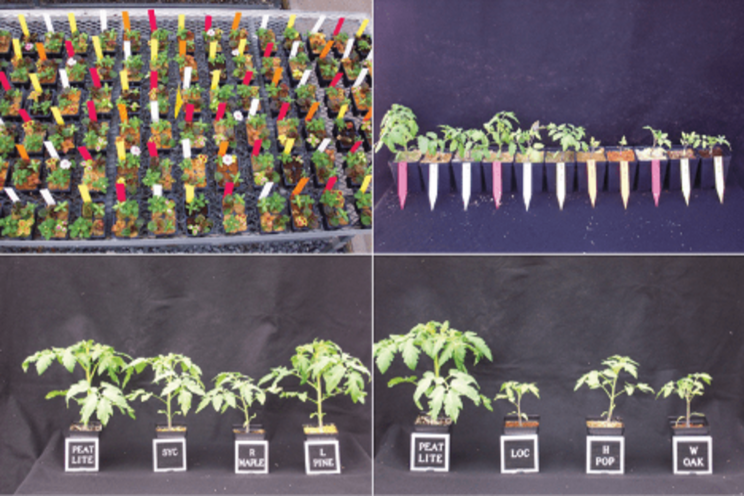
Figure 1: Cross-section of a pine tree illustrating the bark, sapwood and heartwood regions of a tree (A), large pine chips (B), small pine chips (C) and engineered wood fiber (D).

Figure 2: Vinca (A), marigold (B) and tomato (D) plants exhibiting root-growth retardation due to green-wood chemical inhibitors in the substrate; organic acids from various substrate materials often result in colored leachate draining from containers (C).
As the adoption and utilization of soilless substrates that contain wood components has risen in recent years, so has the confidence of many growers who are now exploring the use of higher wood percentages in their mixes and/or experimenting with making their own wood substrates. With these scenarios playing out around the country, challenges can arise. Notably, the use of sawdust materials, wood from hardwood trees and freshly harvested and processed (hammer-milled) pine trees. We will not discuss commercialized wood-fiber materials made from disc-refining or screw-extruded processes in this article, but those will be discussed in the upcoming companion article.
Identifying a phytotoxic (poisonous to plants) plant-growth response is not always easy or clear. When crops are stunted, off-color, exhibit slow or no shoot growth, have reduced rooting or display other abnormal behavior, there are several possible culprits in addition to phytotoxicity, including fertility, pH, substrate physical properties (disproportionate air or water in the rootzone) or non-substrate environmental/ambient factors. In general, one can assume that growth retardation in the germination phase of seed sown crops, rooting phase (for cuttings) or days following plug/liner transplant are associated with phytotoxicity. Granted, this is not always the case, but as a rule of thumb this usually helps separate potential substrate-toxicity effects from nutritional or other growth-reducing factors.

Figure 3: Laboratory and greenhouse testing of substrate phytotoxicity including (A) root growth in mini-horhizotron, (B) seed-germination tests, (C) aqueous-extract seed-germination tests and (D) aqueous-extract, sand-culture bioassays.

Figure 4: Growth of begonia, coleus, impatiens and vinca in substrates produced from 11 tree species.
Wood chemistry is an extremely complex (and daunting) topic to understand, much less discuss! A look through literature identifies many terms used in relation to the chemical constituents of wood and their effect on plant growth, like allelopathy, volatile organic acids (VOCs), tall oil, pitch, extractives, exudates, green wood toxicity, etc. There are many chemical compounds in trees that serve a variety of purposes when the tree is still standing but have other functions or consequences once it is harvested. Known as secondary metabolites, compounds such as flavonoids, lignans, terpenes, phenols, alkaloids, sterols, pectins, waxes, fats, tannins, suberins, resin acids and carotenoids can be present in processed wood meant for use as a substrate or substrate component. Higher concentrations of these compounds most often occur in the bark, heartwood and roots of trees. Bark, the outside protective layer of trees, is different structurally and chemically than sapwood (functioning wood closest to the bark) and heartwood (nonfunctioning wood in the center of trees) as illustrated in Figure 1A. When trees are chipped and further milled, or refined into substrate aggregates or fibers, the release of volatile and water-soluble wood chemicals will increase (Fig. 1B-D). Variation can also occur among species, from tree to tree within in stand, and from season to season during the year.
Over the years, many examples of phytotoxic effects have been seen both in research trails as well as at grower operations that utilize fresh pine wood in their mixes. Not exclusive to seeds and young plants, "sour" or toxic wood mixes have inhibited root growth of annuals (see Fig. 2). Testing of the substrates showed high pH and strong odors of "sour" wood volatiles. No chemical testing was conducted at that time, but the substrate was put in mini-horhizotron root-growth boxes and tested with tomato with similar results in the retardation of root growth (Fig. 2D). Another observation seen frequently when using hardwood-substrate materials is dark-colored leachate draining from containers after irrigation (Fig. 2C). These dark colors originate from water-soluble organic acids in the substrate materials, but no evidence of toxicity is known. The type of wood (or other organic component) as well as percent, age and pH of substrate contribute to the occurrence of these leachates.

Figure 5: Wood substrates derived from different tree species are shown to have growth-reduction potential in various annuals and tomato plants.
Testing for substrate phytotoxicity can involve several quick and easy laboratory and greenhouse procedures. These testing methods have traditionally been used on substrates to test for compost maturity, heavy metal content and salinity, but they also can be used to test for phytotoxic effects of wood substrates. In the Horticultural Substrates Laboratory at North Carolina State University, we use the mini-horhizotron to observe, measure and photograph seed germination, and the resulting root emergence and development, which is a growth parameter found to be a more accurate and sensitive indicator of phytotoxicity than germination alone (Fig. 3A). Other test methods include greenhouse-seedling germination tests followed by weeks of plant growth with and without supplemental fertilizer (Fig. 3B); seed germination and radicle emergence in controlled-environment chambers using aqueous extracts from the substrates (Fig. 3C), and sand-culture bioassay's using wood extractives (Fig. 3D).
Research trials over the past 15 years have evaluated the plant-growth response, phytotoxicity effect and quantification of phenols and other major secondary metabolites on a dozen tree species (Fig. 4). Using species known to be sensitive to phytotoxicity including radish, cress and mustard, as well as annual species such as impatiens, vinca, coleus and begonia, were used in these trails. Results showed severe stunting of many species when grown in wood substrates derived from hardwood species, and to a lesser extent, softwood tree species. Of the softwood species tested, loblolly pine had the least severe growth retardation and germination inhibition, and the lowest overall poly-phenolics concentration. Additional experiments using the same tree species were conducted on tomato in both sand and substrate bioassay trails to evaluate the persistence of phytotoxicity over time and at various plant-growth stages (Fig. 5). Results of all trials were consistent in that when used fresh, all species exhibited some degree of phytotoxicity compared to tap water or peat-based substrate controls. From these trials, efforts were then undertaken (and still are being investigated) to find ways of reducing or removing toxins from fresh wood (specifically loblolly pine).
Based on current knowledge, loblolly pine is the best tree species for making wood substrates, but fresh hammer-milled pine wood (or lumber-mill sawdust) cannot be used alone or at high percentages like other substrate components without potential plant-growth problems. It is critically important for anyone using these materials to practice caution when choosing to use them fresh, and ideally explore ways to reduce the toxicity before use.
The second article in this series will address methods of mitigating potential phytotoxic effects on greenhouse crops grown in wood substrates. Both proactive and reactive methods of reducing or eliminating wood toxins will be discussed, including wood processing methods, wood feedstock and processed-substrate preconditioning treatments, substrate additives and employment of various cultural practices during crop production.
The author is an associate professor and director of the Horticultural Substrates Laboratory at North Carolina State University. Reach him at Brian_Jackson@ncsu.edu
Source and Photo Courtesy of Greenhouse Management
Source: Greenhouse Management
More news















